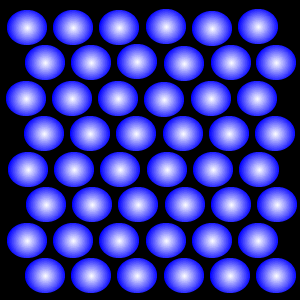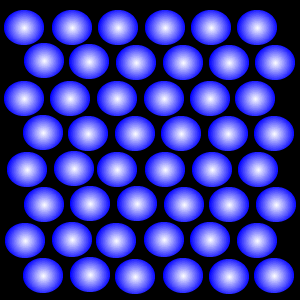
Particles in the solid state are closely packed and can vibrate but cannot move around, they have low energies. Solids therefore have their own shapes because the particles do not flow. Because the particles are so close together, solids cannot be compressed (squeezed into a smaller volume).
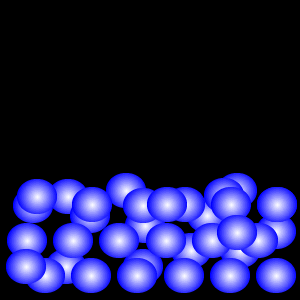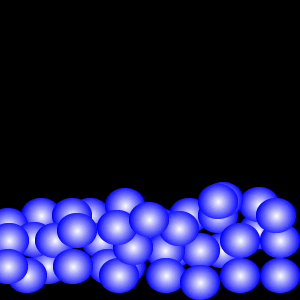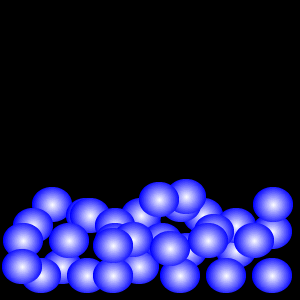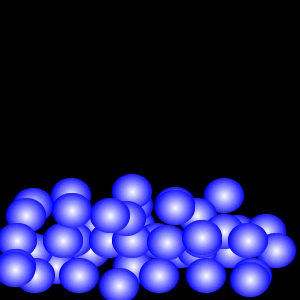
Particles in the liquid state are still closely packed, but can both vibrate and move around within the liquid because they have more energy – enough to overcome the forces that hold the particles together in the solid. For this reason the particles in a liquid can flow, and a liquid will take up the shape of whatever container it is placed in. Because the particles in a liquid are still close together, liquids cannot be compressed.
Particles in the gaseous state are widely spread out and can both vibrate and move around freely. They have the most energy of the three states. The particles can go anywhere within their container, and so gases have no shape. Because the particles are so far apart, they can be squeezed into a smaller volume, i.e. compressed. This increases the pressure of the gas.

What are these “particles”?
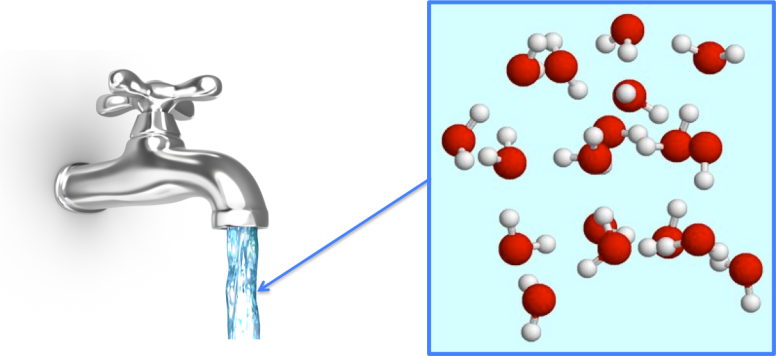
In some substances, they could be individual atoms e.g. in noble gases such as helium or argon. More usually they are groups of atoms bonded together, each group of atoms being the same. We call these molecules.